ALGS Part 2: The Mechanism - Why ALG-000184 Might Actually Close the Trapdoor
Capsid modulators have failed before. The graveyard is full of promising molecules that couldn't escape resistance or deliver antigen reductions. So what makes Aligos think they've solved it?
1) Why Every CAM Before This Failed
The first generation of capsid assembly modulators looked promising in the lab. They disrupted HBV's ability to package its genetic material, achieving rapid viral suppression in early trials. But the human body is a harsher testing ground than a petri dish.
The resistance problem hit fast. JNJ-56136379 (bersacapavir) - Janssen's lead CAM with backing from one of the world's largest pharmaceutical companies - showed strong initial suppression. Within months, patients began developing resistance mutations. The virus adapted. Suppression faded. The program was discontinued.
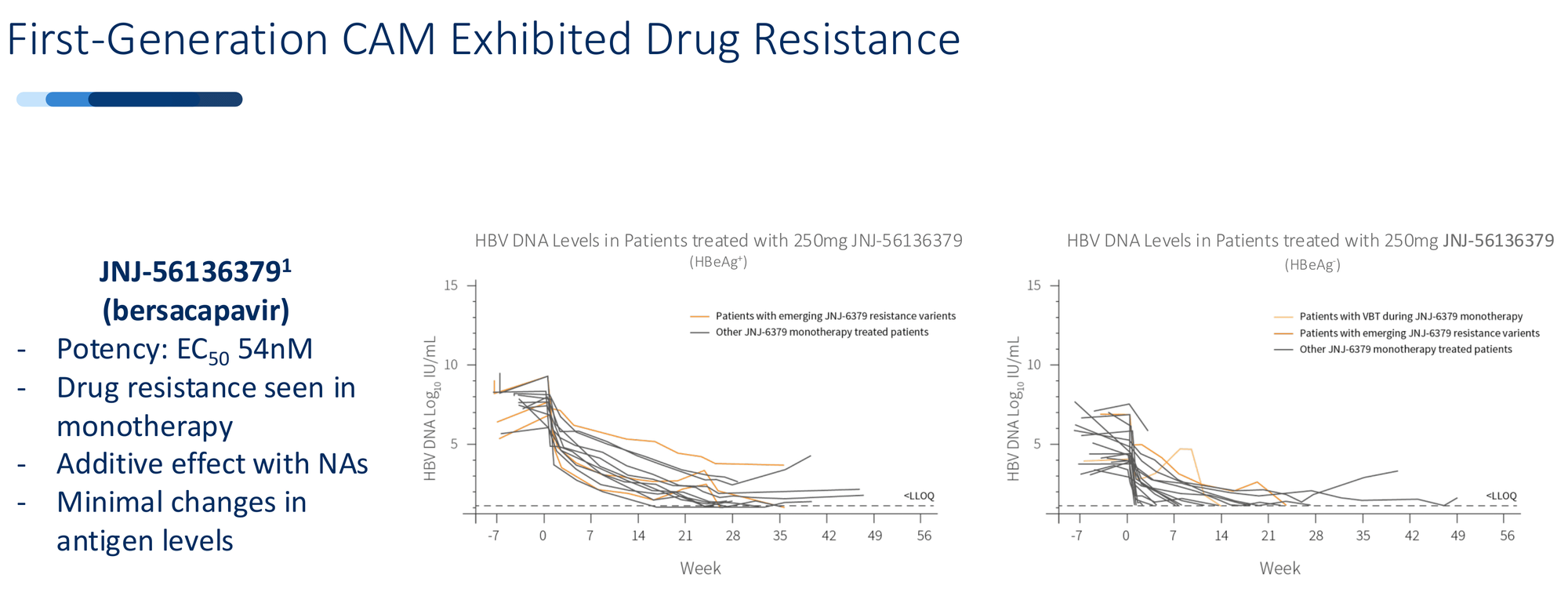
Vebicorvir from Assembly Biosciences followed the same path. Strong preclinical data, promising Phase 1 results, then resistance mutations emerged in longer dosing. The FDA wouldn't allow a chronic suppression pathway for drugs showing resistance as monotherapy. Without that regulatory route, the commercial case collapsed.
The pattern was clear: first-generation CAMs could suppress the virus temporarily, but they couldn't prevent the evolutionary escape. And they barely touched the antigen burden - the massive reservoir of viral proteins that exhausts the immune system and prevents functional cure.
The mechanism was too narrow. Suppress replication, but leave everything else intact.
2) The Two-Step Kill
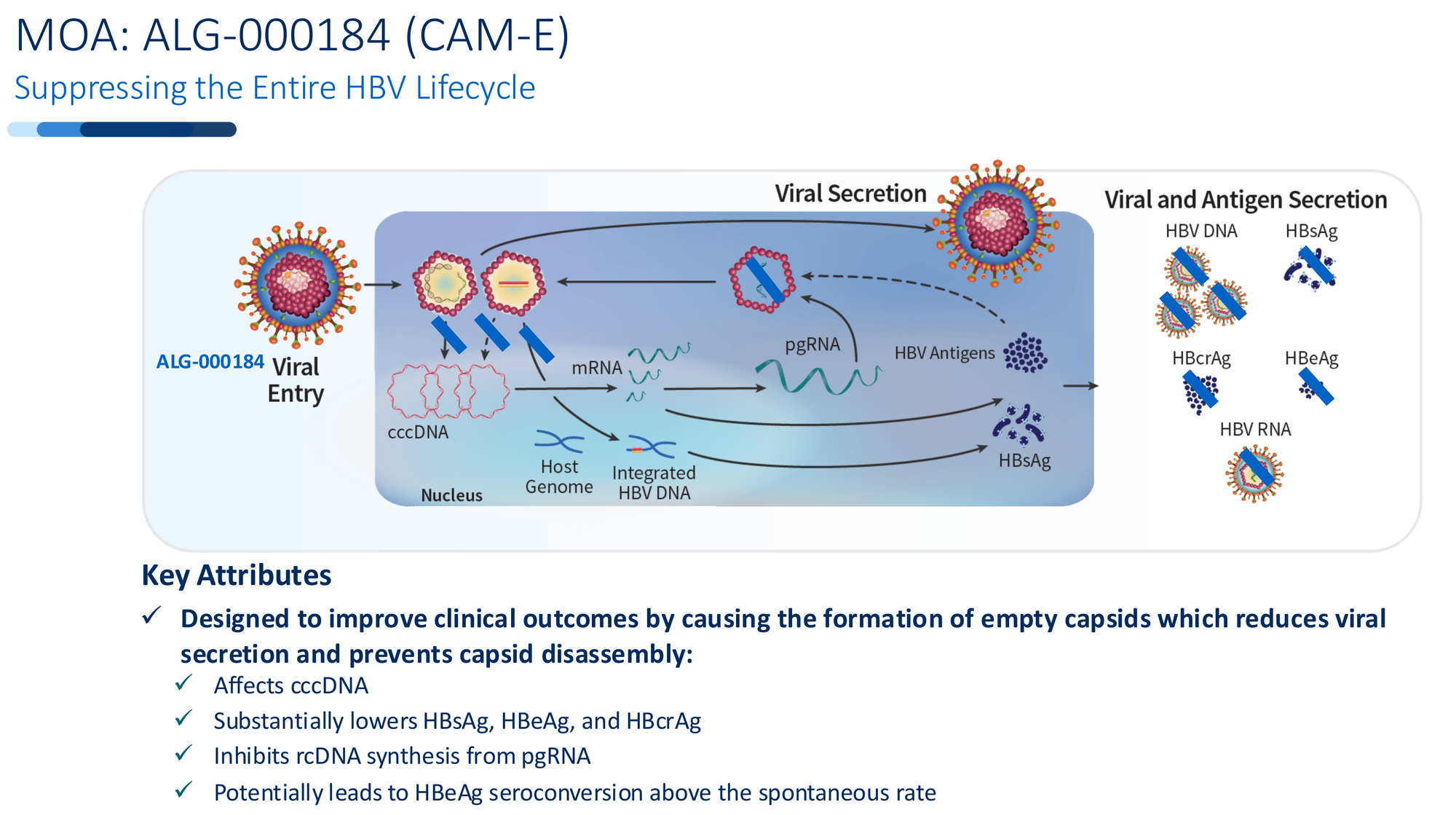
ALG-000184 was designed with a different ambition: attack the entire viral lifecycle, not just one piece.
Step 1: Empty Capsid Formation
Most antivirals, including standard nucleoside analogs, target the virus after it's already assembled. ALG-000184 intervenes earlier - during capsid assembly itself. It forces the virus to create empty, malformed capsids that can't package genetic material. No pgRNA gets in. No new virus gets made.
This delivers immediate, profound suppression. HBV DNA drops rapidly because the assembly line breaks down at the source.
Step 2: Blocking the Trapdoor
But here's where it diverges from failed predecessors. Those empty capsids can't just be discarded. Under normal infection, functional capsids disassemble in the nucleus and deliver their DNA payload to replenish cccDNA - the master template that regenerates the virus indefinitely.
ALG-000184-induced capsids can't disassemble properly. They can't deliver new cccDNA. Over time, the existing cccDNA reservoir isn't replenished. It begins to decay.
This is the trapdoor mechanism. Not just suppression - actual depletion of the source.
And crucially, this dual mechanism creates a high genetic barrier to resistance. The virus would need multiple simultaneous mutations to escape both the capsid disruption and the cccDNA block. In 96 weeks of human dosing, that hasn't happened.
3) The 96-Week Proof
Preclinical promises mean little in HBV. The field is littered with molecules that worked beautifully in cell culture and failed in humans. What separates ALG-000184 is unprecedented durability in extended human dosing.
100% viral suppression. Every patient in the 96-week monotherapy cohort - both HBeAg+ and HBeAg- - achieved HBV DNA below the limit of quantification. Not 90%. Not 95%. All of them.
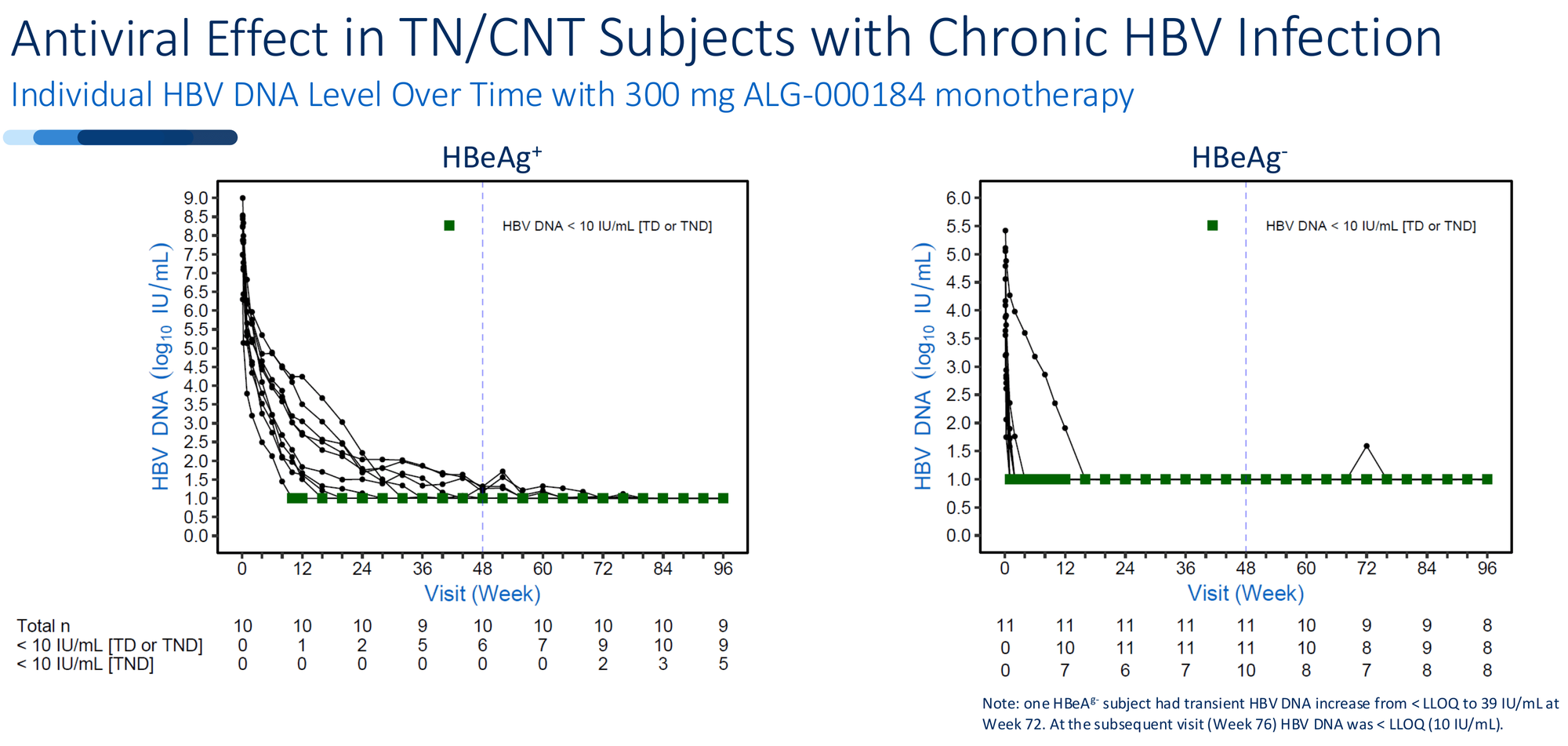
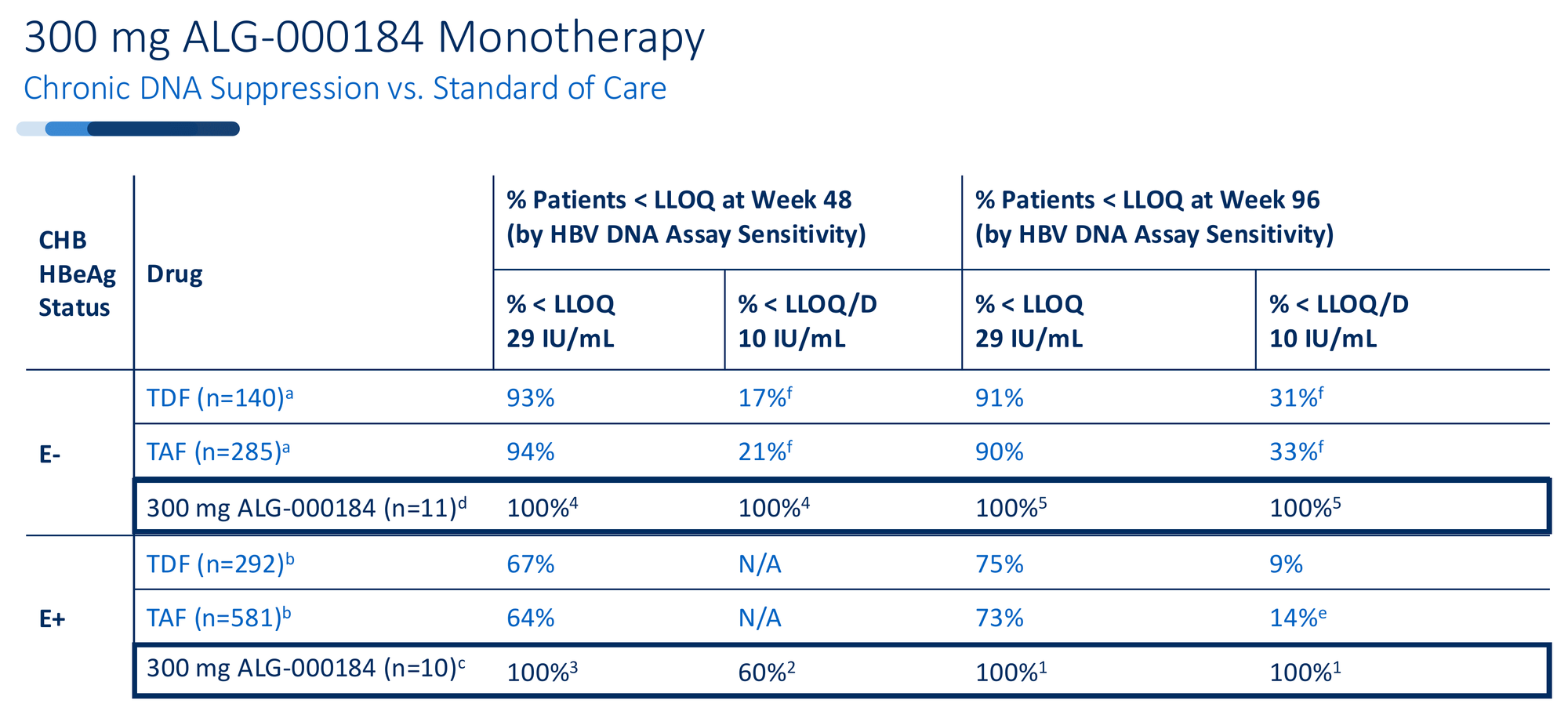
Compare this to tenofovir, the current standard of care. At 96 weeks, tenofovir achieves undetectable HBV DNA in roughly 30-33% of patients when measured with sensitive assays. ALG-000184 in early data: 100%.
But suppression alone isn't enough. The real signal is in the antigens.
Multi-log antigen reductions - something nucleoside analogs simply don't deliver. HBsAg declined by ~1 log in HBeAg+ patients. HBeAg dropped by ~2 logs. HBcrAg - a marker of cccDNA transcriptional activity - fell by over 2 logs.
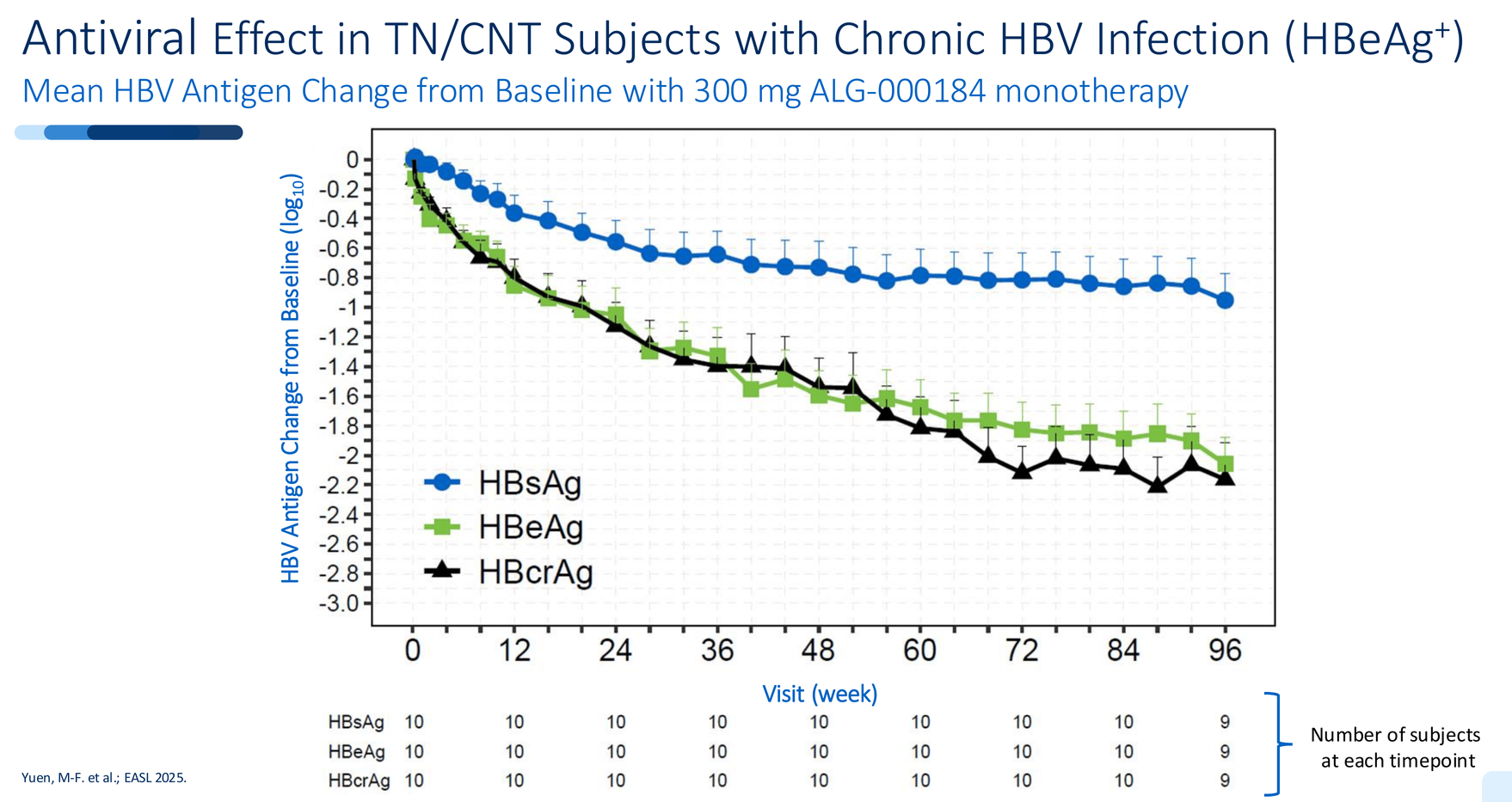
This isn't incremental improvement. Tenofovir barely moves these markers. Bersacapavir showed minimal antigen impact before resistance hit. ALG-000184 is doing both: complete suppression and meaningful antigen decline simultaneously.
Zero resistance mutations. Deep sequencing through 96 weeks found no emergence of ALG-000184-specific resistance mutations in any patient. The major resistance mutations seen with prior CAMs (T33N, T33P, V124G, R127H) - absent.
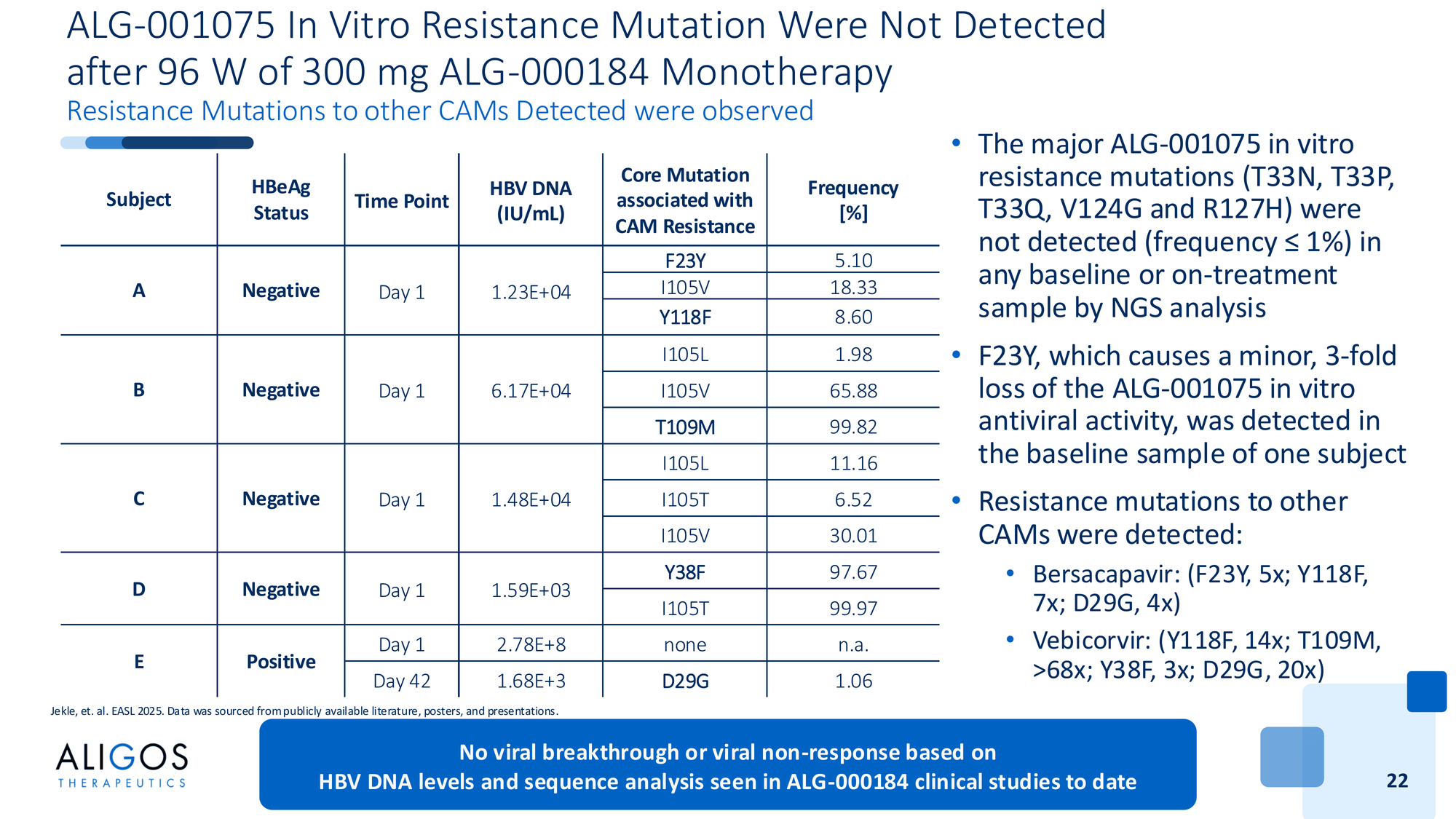
Interestingly, the sequencing did detect resistance mutations to other CAMs present at baseline - mutations that confer resistance to bersacapavir and vebicorvir. But those mutations don't affect ALG-000184. Different binding, different resistance profile.
This is the data set no other oral therapy has produced: sustained suppression, antigen decline, and no resistance through nearly two years of monotherapy.
4) The Moment of Truth: Post-Treatment Follow-Up
Here's the billion-dollar question: what happens when you stop?
Functional cure in HBV is defined by sustained HBsAg loss after stopping treatment. Not just suppression while on therapy - durability after you discontinue. This is what separates a better chronic therapy from a potential cure pathway.
The 96-week dosing has completed. Patients have now entered post-treatment follow-up. The data will be presented at AASLD in November 2025.
Three scenarios are possible:
Scenario 1: Sustained Durability
HBV DNA remains undetectable. Antigens stay suppressed or continue to decline. cccDNA depletion proves durable. This would be the first signal that an oral monotherapy might enable functional cure in at least a subset of patients. The investment case transforms immediately.
Scenario 2: Partial Durability
Some patients maintain suppression, others experience gradual viral rebound. This suggests patient selection matters - perhaps baseline antigen levels, HBeAg status, or treatment duration determine outcomes. Still valuable, but requires combination approaches for broader efficacy.
Scenario 3: Rapid Rebound
HBV DNA returns quickly in most patients once treatment stops. The cccDNA reservoir wasn't depleted enough. ALG-000184 proves to be an excellent suppressive therapy - potentially superior to tenofovir - but not a cure enabler as monotherapy.
Even Scenario 3 isn't failure. A therapy that achieves 100% suppression with no resistance and significant antigen reduction still has enormous value. But Scenarios 1 and 2 open the door to something bigger.
November 2025 will tell us which path we're on.
5) B-SUPREME: The Superiority Gamble
Most companies developing HBV therapies run non-inferiority trials. Show you're as good as tenofovir, file for approval, avoid the risk of head-to-head failure.
Aligos chose a different design: superiority.
The B-SUPREME trial randomizes roughly 200 treatment-naïve patients 1:1 to receive either ALG-000184 monotherapy or tenofovir for 48 weeks. The primary endpoint: proportion of patients achieving HBV DNA below the lower limit of quantification (target detected or not detected) at Week 48.
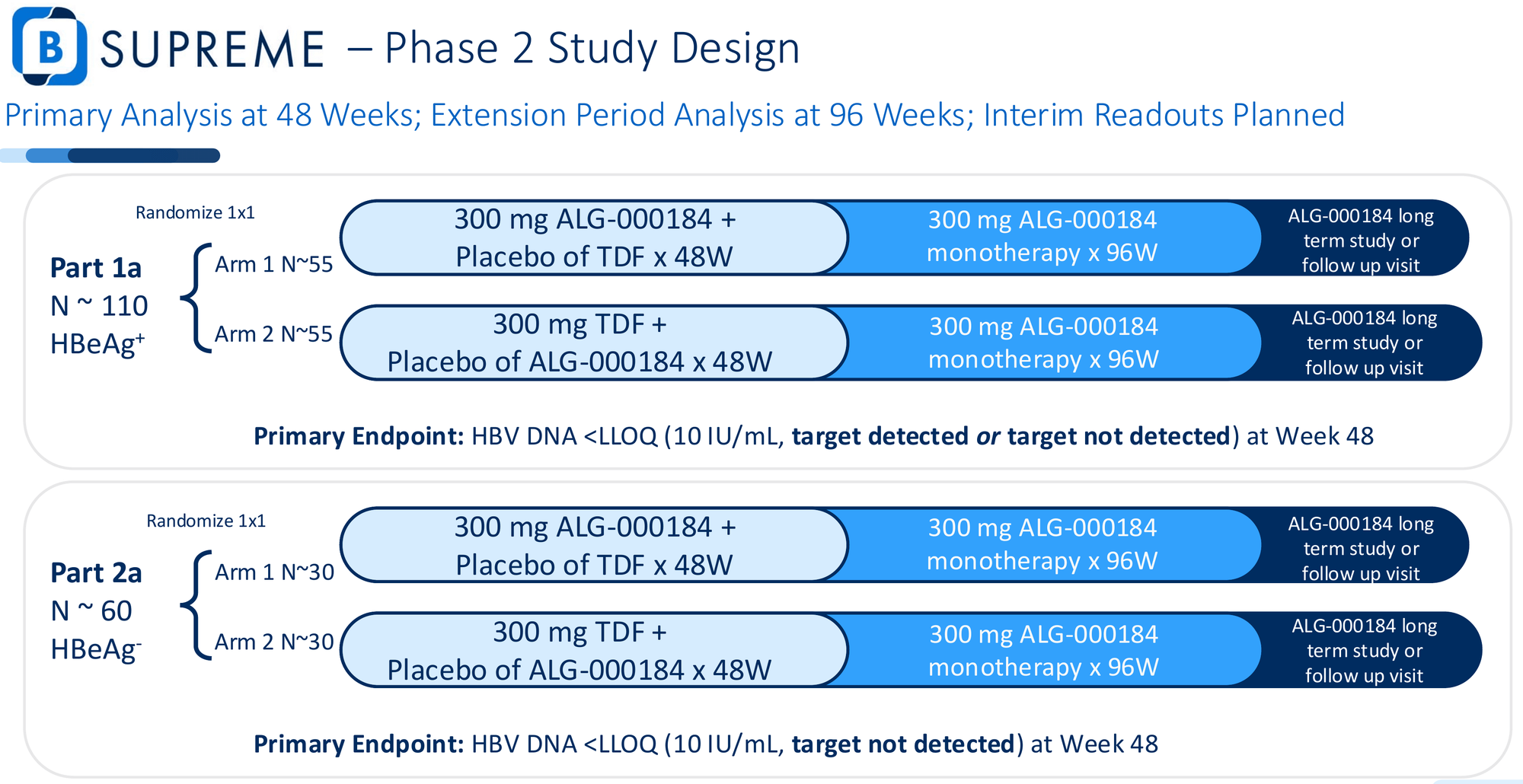
This is a high-stakes design. Non-inferiority would have been safer - easier to hit, lower bar for approval. Superiority means ALG-000184 must statistically outperform the global standard of care.
Why take that risk? Because the Phase 1 data suggests they can win.
If ALG-000184 delivers 100% undetectable HBV DNA at 48 weeks while tenofovir achieves 60-70% (the historical range for HBeAg+ patients), that's not just statistical significance - that's a paradigm shift. Payers would have to reconsider formularies. Physicians would have a new first-line option. The "better mousetrap" argument becomes undeniable.
What success looks like:
- Primary endpoint: Significantly higher proportion of patients with undetectable HBV DNA vs. tenofovir at Week 48
- Key secondary endpoints: Antigen reductions (HBsAg, HBeAg, HBcrAg), ALT normalization, resistance surveillance
Timeline:
- Interim data: 2026 - First look at comparative efficacy
- Full 48-week topline: 2027 - Primary endpoint readout
- 96-week extension data follows for durability signals
If the interim data in 2026 shows clear separation from tenofovir, the narrative shifts before the trial even completes. If it shows equivalence or inferiority, the market will reprice risk immediately.
The superiority design is a declaration of confidence. It's also a bet that the 96-week monotherapy data wasn't a fluke.
But here's the strategic insight most investors are missing: ALG-000184 doesn't have to win as monotherapy to create massive value. The real opportunity lies in what it enables when combined with other approaches - and Aligos has been quietly building that optionality.
In Part 3, we'll explore the combination play: why ALG-000184 might be the backbone every RNA program needs, and how Aligos positioned itself to win regardless of which cure approach succeeds.
Disclaimer
The information provided on this website is for informational purposes only and should not be construed as
financial, investment, legal, or professional advice. While efforts are made to ensure accuracy, no guarantee
is given regarding completeness or reliability. Visitors should conduct their own research or consult a qualified
advisor before making any decisions. External links are provided for convenience and do not imply endorsement.